Sprint Pns System Side Effects
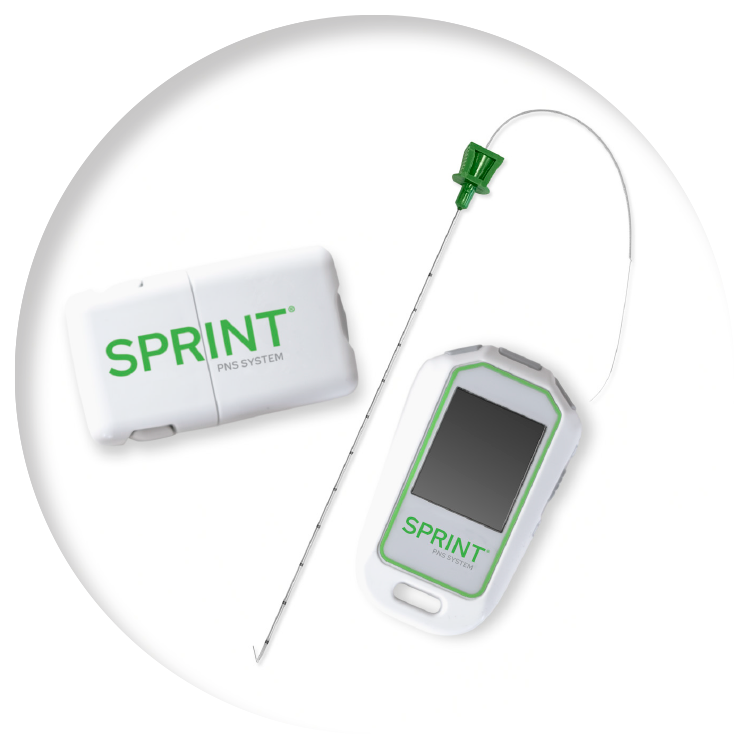
Sprint pns system side effects. The leads attach to devices worn on your body that deliver stimulation called Stimulators. FDA 510k clearance for the treatment of chronic pain and acute pain including postoperative and post-traumatic pain. Symptomatic relief of post-traumatic pain.
Additional stimulation side effects were documented. The risk of biologic complications is usually immediate. For more information see the SPRINT PNS System IFU.
For more information see the SPRINT PNS System IFU. Symptomatic relief of chronic intractable pain post-surgical and post-traumatic acute pain. The most common side effect was skin irritation or redness at the lead exit site or related to the use of adhesive patches or bandages.
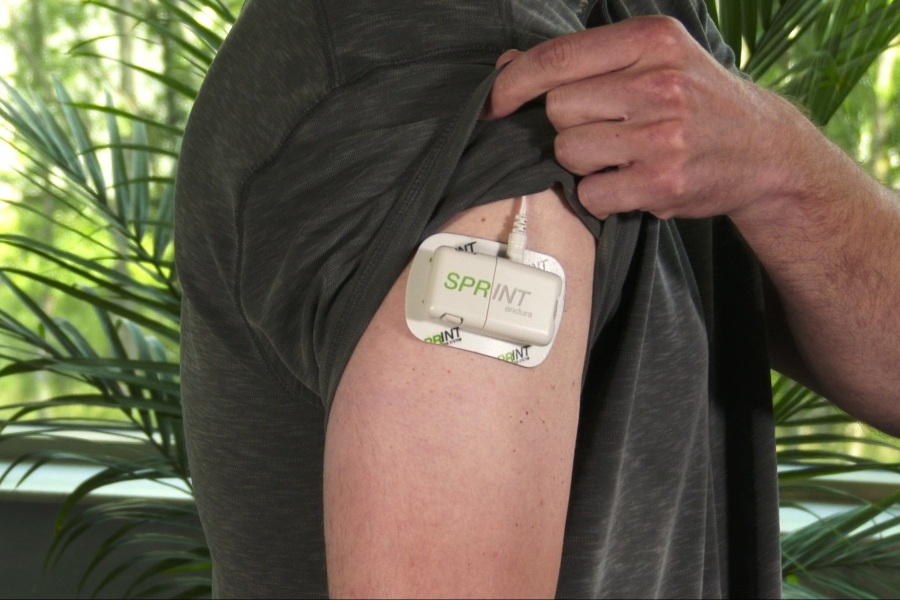
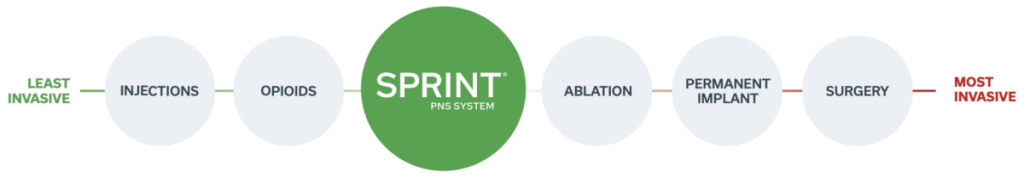
It also includes a device worn on the body that delivers stimulation called the SPRINT Stimulator. The SPRINT PNS System is not intended to treat pain in the region innervated by the cranial and facial nerves. Most common adverse events are skin irritation and erythema.
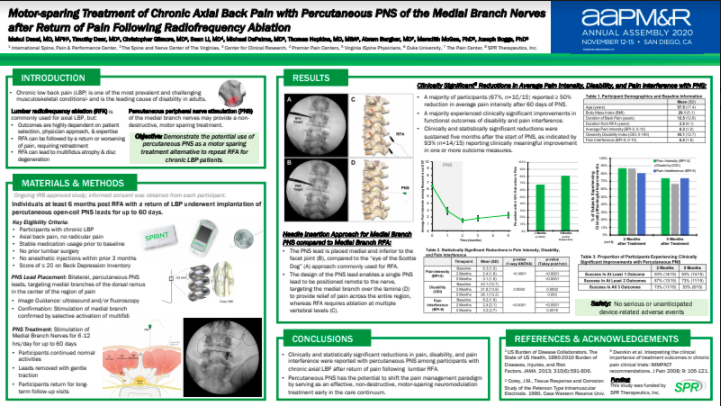
Sprint peripheral nerve stimulation system. Furthermore opioid use among moderate to high users which represented about a quarter of the participants decreased by more than 70 in the SPRINT PNS group compared to less than one percent in the placebo group. Most common adverse events are skin irritation and erythema.
I have had great success in utilizing the SPRINT PNS System for many of my patients and Im pleased that the expanded indication allows me to now treat these areas of high interest in an on. SPR Therapeutics says 74 of patients treated with the Sprint PNS reported a greater than 50 reduction in pain. 10 SPRINT PNS System Patient Instruction for Use L0092-MAN-000 Rev F The SPRINT System is MR unsafe All MRI procedures anywhere on your body are not allowed while you are using the SPRINT System.
A nerve block is not required before a PNS system is tried but your doctor may recommend one. Technical complications usually occur within 2 years of implant.
I have had great success in utilizing the SPRINT PNS System for many of my patients and Im pleased that the expanded indication allows me to now treat these areas of high interest in an on.
Sprint Pain Nerve Stimulation PNS System Videos - Last updated on. Most common adverse events are skin irritation and erythema. The SPRINT System is an Investigational Device which delivers mild electrical stimulation to nerves in the leg that underwent lead placement. SPR Therapeutics says 74 of patients treated with the Sprint PNS reported a greater than 50 reduction in pain. For more information see the SPRINT PNS System IFU. The leads attach to devices worn on your body that deliver stimulation called Stimulators. Physicians should use their best judgment when deciding when to use the SPRINT PNS System. Most common adverse events. Sprint peripheral nerve stimulation system.
The SPRINT PNS System is indicated for up to 60 days for. Sprint Pain Nerve Stimulation PNS System Videos - Last updated on. Patients who want the treatment may have to battle their. Most common adverse events are skin irritation and erythema. The SPRINT PNS System is not intended to treat pain in the region innervated by the cranial and facial nerves. Your doctor may also suggest a psychological evaluation to identify factors that could affect the outcome of PNS such as depression anxiety and personality disorders. Physicians should use their best judgment when deciding when to use the SPRINT PNS System.



































Post a Comment for "Sprint Pns System Side Effects"